–by Anthony Zukoff – Entomology Southwest Research and Extension Center
Garden City, KS
Insect diagnostic services are available to members of the public seeking assistance identifying an insect or suspected insect related problems. The Insect Diagnostics ID Request Form can be accessed online and after providing observation information such as location and date of the sighting along with answering a set of questions intended to help with the identification process, one can then upload up to 3 photos and submit the form. The inquiry is then forwarded on to one of the entomology extension specialists. Within a few days, usually less than two, the identity of the insect along with appropriate life history information and/or control measures is then sent to the client by email or phone. The online submission process takes only a few minutes and can be accessed with desktop computers and mobile devices.
During the 2022 season, Insect Diagnostics has processed 54 inquiries from 2 states. Identification requests fell into several categories, from requests out of general curiosity to much more specific identification needs. The Home/Structural and General categories contained the bulk of the season’s inquiries (Figure 1). During the season, a variety of clientele reached out to our program for identification assistance. Homeowner’s submitted the most requests, however, government entities, commercial pest control and horticultural services utilized our service as well (Figure 2).
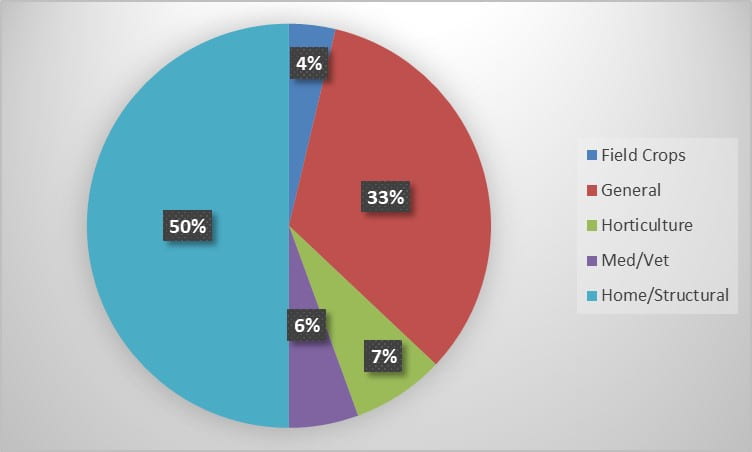
Figure 1. Percent of total inquiries received for each request category during the 2022 season.
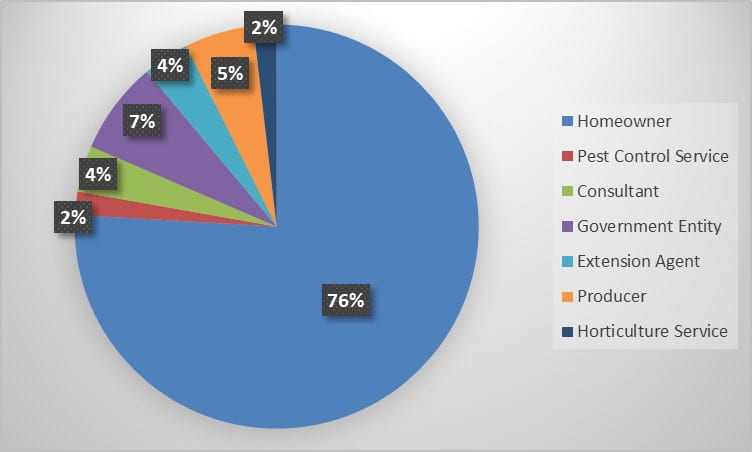
Figure 2. Percent of total inquiries received from each clientele category during the 2022 season.
Insects identified this season varied greatly. The colorful nymphs of green stink bugs caught quite a few homeowners’ eye this year and several instances of Kissing Bugs being found in homes caused concern (Figure 3). The end of the season was dominated by many homeowners requesting identification of elm leaf beetles which have begun searching for overwintering spots inside homes and barns.

Figure 3. One of several Triatomine bugs (“Kissing Bugs”) submitted this year. A vector of Chagas Disease in extreme southerly locations of the United States and the tropics, but not of concern in Kansas.
The main season for insect activity may be ending, but the Insect Diagnostics Program will continue to operate and accept online inquiries throughout the fall and winter. If you need insect identification assistance, submit a request at https://entomology.k-state.edu/extension/diagnostician/.

































