–by Jeff Whitworth – Field Crop Entomologist
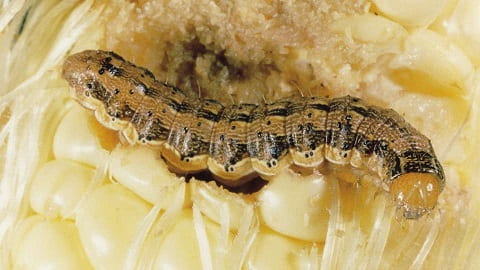
Adult fall armyworms are moths (fig. 1) and have been reported from southern Kansas for about the last 2 weeks and probably will be in the rest of the state soon-if not already. A quick refresher relative to this pest: they normally do not overwinter in Kansas, but in the lower portions of southern states, and down into Mexico and Central America. The moths usually arrive in Kansas anywhere from mid-June to mid-July as they fly/are blown here on southern winds. Fall armyworms have a wide host range but in Kansas are most often a cause for concern in corn and/or sorghum and later sometimes in wheat depending on planting date and weather. Also, in Kansas, especially the last 2 years, brome has been seriously defoliated (in combination with armyworms) in many areas around the state. Armyworm larvae may be part of the “ragworm” complex of larvae feeding in the whorl of corn and/or sorghum and then later the next generation become part of the complex of “headworms” or larvae feeding in the sorghum head directly on the developing grain. So the moths are here, ovipositing and thus the larvae are, or soon will, be feeding on whatever host the eggs were deposited on. Fall armyworms were quite common (in combination with other species) in 2020 and 2021 and thus monitoring should be initiated in any potentially susceptible crops. For management considerations please refer to the 2022 KSU Insect Management Guide for the crop of interest.
(Photo of fall armyworm moths taken of a Riker mount provided by revered KSU Extension entomologist, now retired, Dr. Bob Bauernfeind).

Figure 1: Fall armyworm moths