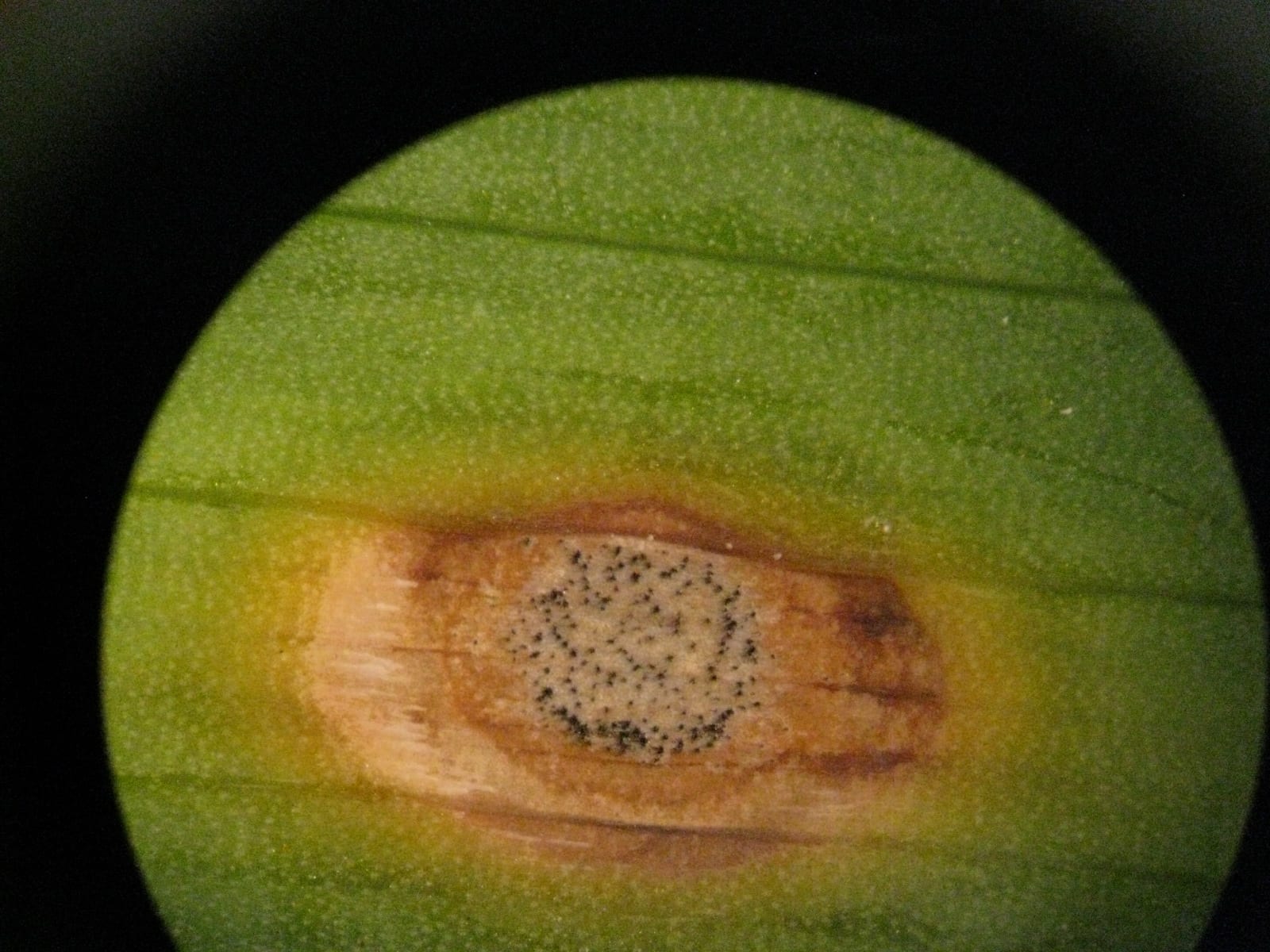
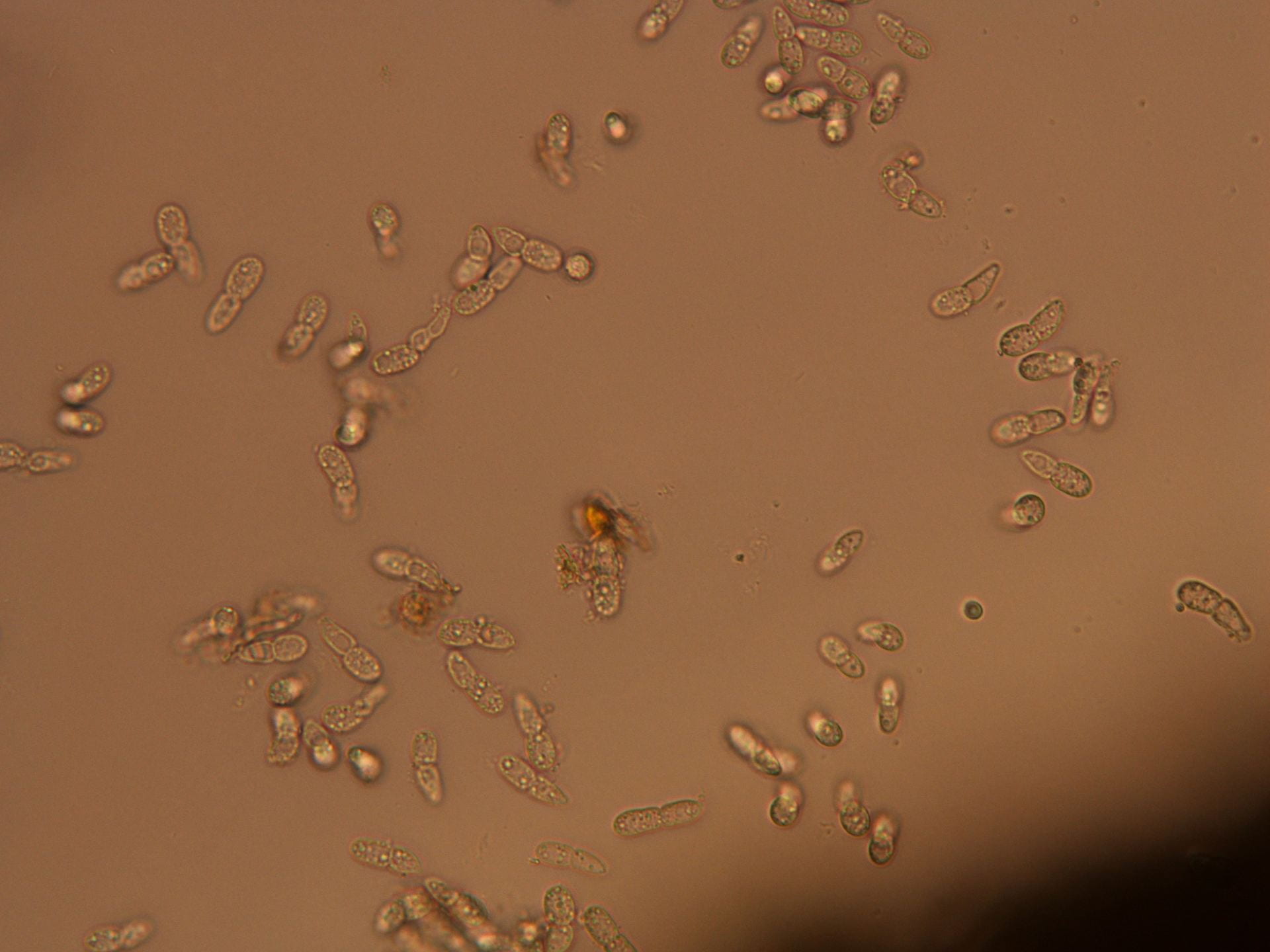
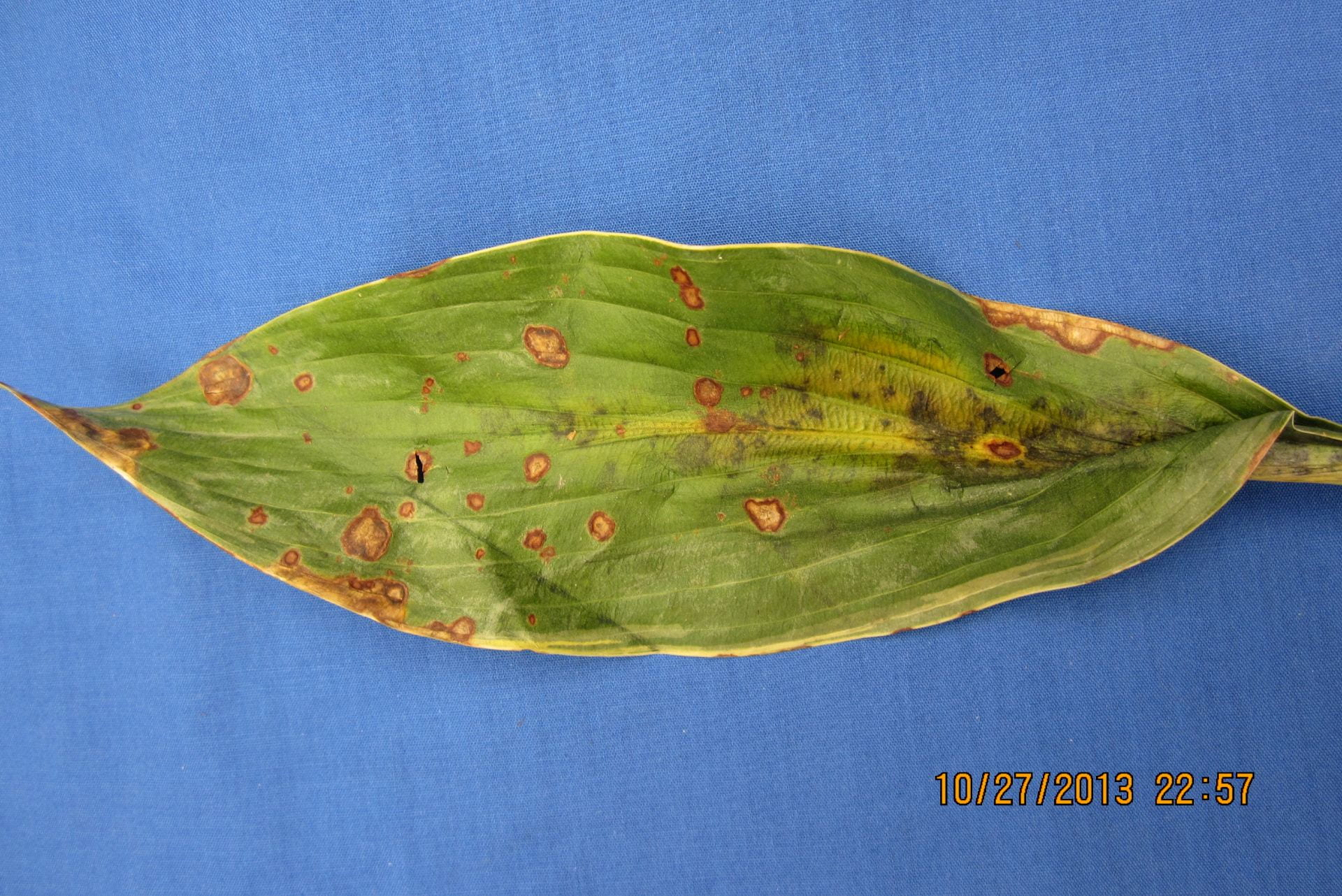
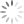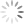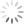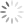(Megan Kennelly, KSU Plant Pathology)
Here is a sample that came into the lab recently:
You can see turf decline, and if you look closely you can see some dark green algae in the brown area. Algae often indicates poor drainage. This site is in a low area with poor airflow. The site has a lot of underlying stress.
Here is a view of the washed-off roots sitting on the dissecting microscope. They should be a creamy white but instead they are more of a brown color. They were mushy in texture as well.
Finally below is a closer view in the compound microscope. You can see how the roots are dark. Healthy roots are much more clear/transparent. These are also lacking fine root hairs, and the outer tissues have sloughed off.
These symptoms occur frequently in sites with poor drainage. The roots sit wet, and oxygen flow is disrupted. The wet soil holds heat overnight as well.
The environmental stress alone can cause major root decline and turf damage.
In addition, these conditions can trigger Pythium root rot. (This particular sample did have some Pythium as well, I just had a hard time getting a clear photo). And, these stress conditions can also lead to crown anthracnose. I’ve seen a couple of samples with that disease lately as well. Anthracnose is more likely to chow down on turf that is already stressed.
Here is a link to a publication I’ve mentioned countless times on this blog:
http://www2.ca.uky.edu/agcomm/pubs/ppa/ppa1/ppa1.pdf
There are excellent sections on individual diseases, but there is also a detailed section about summer stress on page 6. Many of the stress-reducing practices listed there will also reduce susceptibility to diseases.
That publication does not discuss Pythium root rot (PRR). (It does discuss Pythium root dysfunction (PRD) which is related but different.) Here is a great resources on PRR:
https://www.turffiles.ncsu.edu/diseases-in-turf/pythium-root-rot-in-turf/
Each year, we say, “I hope this August isn’t a bad one”. This coming week there will be some lower highs (low to mid 80’s) and “lower lows” (mid-60’s overnight, and even some upper 50’s! Woohoo!). Cool temps will be a blessing. However, continued rain may exacerbate drainage problems.
Managing the diseases is important, but it’s critical to address the physiological/environmental stresses as well or the turf can still suffer major decline this time of year.






 Iris leaf spot is not an easy disease to clean up because it overwinters in the residue. So the first step for management is to clean up the flower bed in the fall after frost has killed the tops. This will help to reduce the amount of disease that is carried over. Unfortunately it won’t get rid of the disease. If the planting is old and crowded, digging them up and respacing them will improve air flow. This can help to reduce disease severity.
Iris leaf spot is not an easy disease to clean up because it overwinters in the residue. So the first step for management is to clean up the flower bed in the fall after frost has killed the tops. This will help to reduce the amount of disease that is carried over. Unfortunately it won’t get rid of the disease. If the planting is old and crowded, digging them up and respacing them will improve air flow. This can help to reduce disease severity.